Vascular Theory Exams 2024
16th July 2024
Author: Dr. Nida Nadeem
Vascular Laboratory, Kings College Hospital London
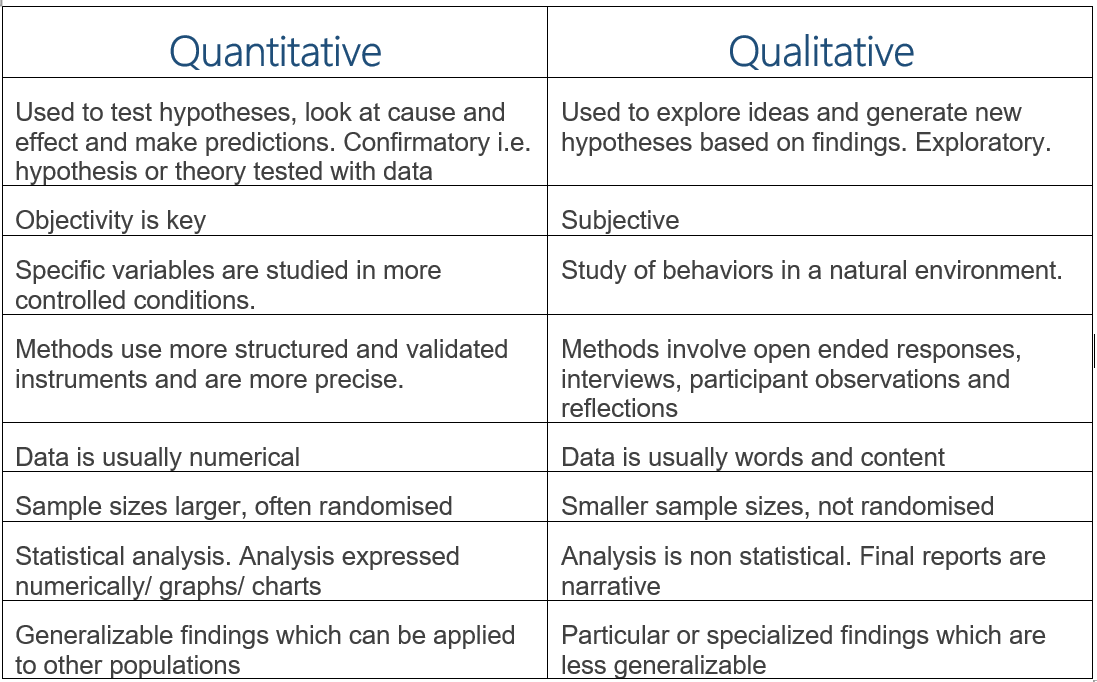
Evidence based research is all about applying the most suitable study design to fit the particular research question. Study design is one of the most crucial aspects of planning a clinical study. You can have the most amazing research question which everyone wants to know the answer to, but without a proper study design, the research might not be considered robust or the conclusions of the study may not reflect the truth. You do not want to weaken your study by not thinking about the study design properly. Many different quantitative and qualitative research designs exist, each with their own strengths and limitations. This research article explores some study design types, when they might be utilized and the strengths/weakness of each.
 |
 |
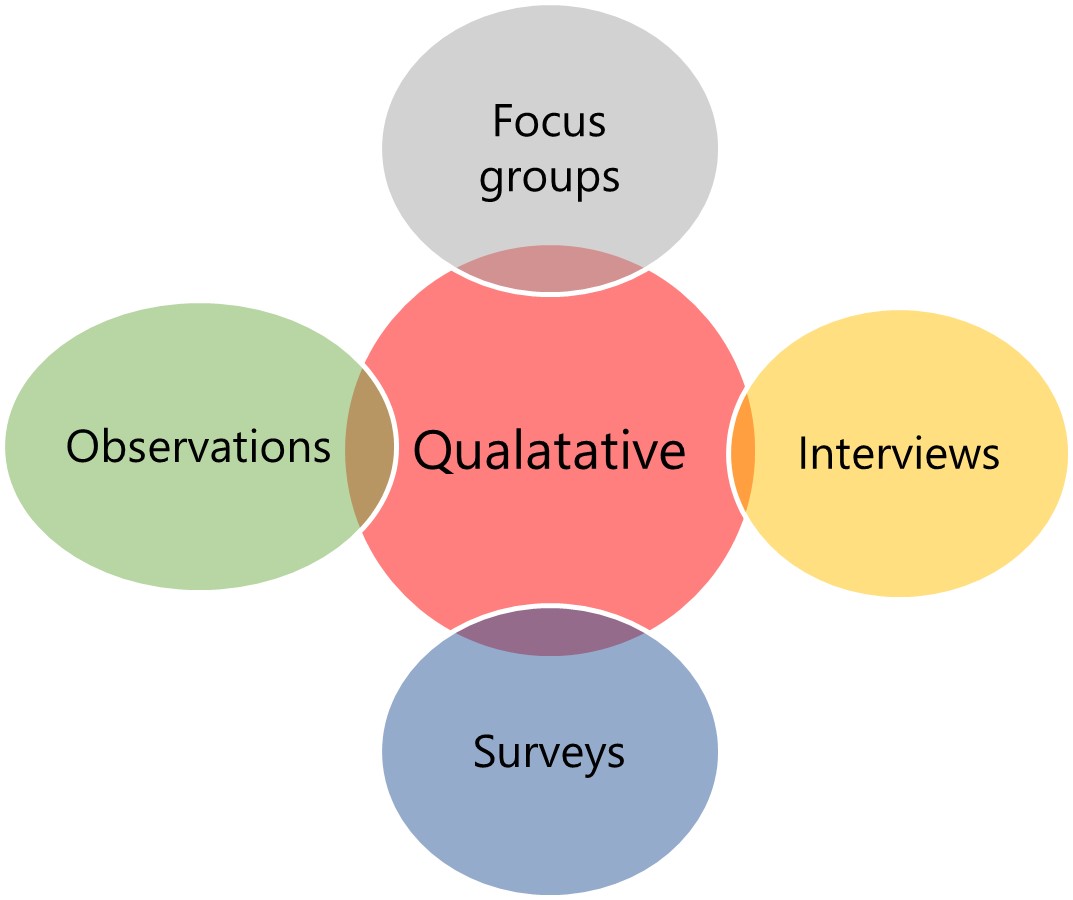 |
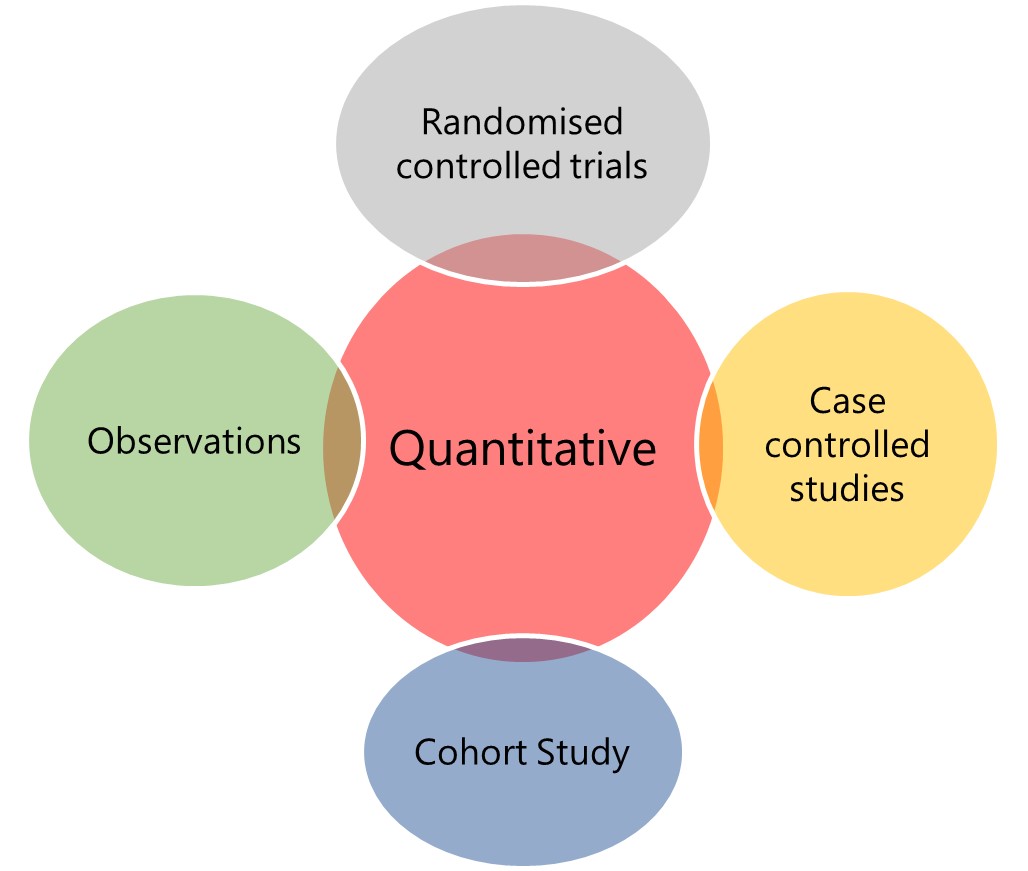
Figure 1: Examples of qualitative and quantitative research studies
RCT is the most robust study design in healthcare to answer questions that evaluate the effectiveness of interventions. The basic structure of a RCT is displayed in figure 2. A RCT is a true experiment in which participants are randomly allocated to receive a new intervention / conventional intervention (experimental groups) or no intervention at all (control group).
The groups are treated and observed identically apart from the actual intervention, therefore any differences in outcome are attributed to the trial intervention.
The process of randomization eliminates selection bias. It should in theory balance out baseline confounding factors, both known and unknown.
RCTs that are blind are considered better to avoid bias in reporting results. There are two types:
Single blind: When the participants do not know whether they are in the control or treatment group, but the researchers do.
Double blind: Neither participants nor researchers know who is in the control or treatment group, this can also include data collectors and statisticians.
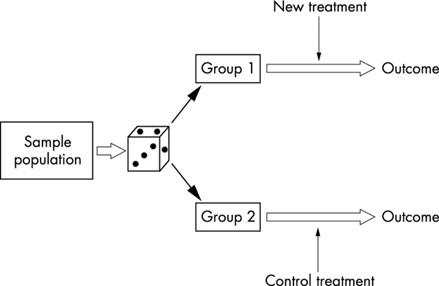 |
Figure 2: Randomized controlled trial study design. Source: Emergency Medicine Journal 2003; 20: 164-168.
The main strengths of RCTs are
Disadvantages of RCTs include:
When the evaluation of an intervention using a RCT is unethical or unfeasible, we can think of using an alternative study design such as a cohort study (figure 3). This study design is similar to the RCT in that there are comparison groups and they are followed up to determine who experiences the outcome of interest. The important difference between the two designs is the absence of random allocation to study groups. Cohort studies can be used to compare two different forms of treatment over the long term.
Cohort defines a population group followed over a period of time. Investigators compare the experience of one group exposed to a factor (exposed group) with that of the other which was not exposed to the factor (control group). If the exposed group has a higher or lower frequency of an outcome than the unexposed, then an association between exposure and outcome is evident. It is important that the study population is free of the disease of interest at entry point into study.
Cohort study is generally an observational study, but it can be either prospective or retrospective. Prospective design allows exposure to risk factors to be assessed directly and confounding variables to be considered. A retrospective design is effective for diseases with a long development time.
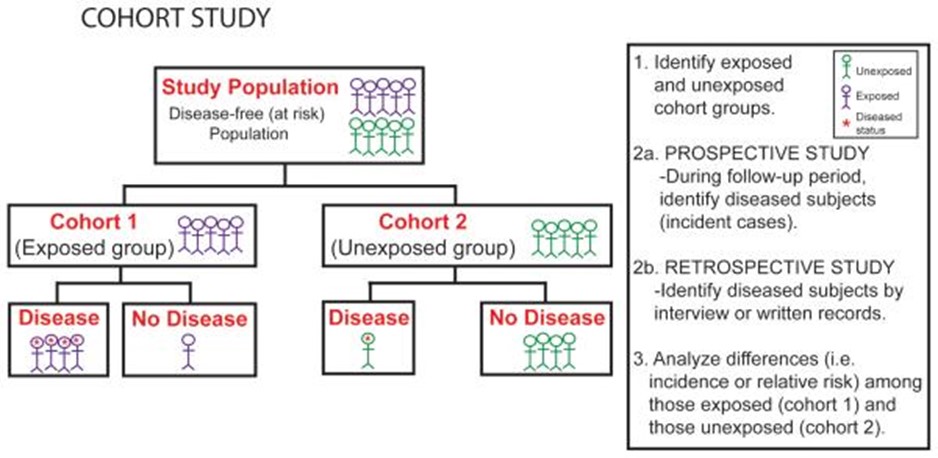 |
Figure 3: Cohort study design. Source: Plast Reconstr Surg. 2010 Dec; 126(6): 2234–2242
Advantages:
Disadvantages:
When the outcome of interest is rare or takes a long time to develop, neither RCTs nor cohort studies may be feasible. In these circumstances it may be better to think about using a case controlled study design. In a case control design, patients with the outcome of interest (cases) and patients without the outcome of interest (controls) are identified and then the investigator determines whether they have had previous exposure to the causative agent (figure 4). The investigator is able to match the case and control patients on variables that may influence the outcome (e.g. age, sex, and other health conditions). This ensures the groups are as similar as possible and the specific effect of the causative agent on the outcome can be more confidently determined. This is usually a retrospective study design type.
Advantages:
Disadvantage:
 |
Figure 4: Case-control study design. Source: Plast Reconstr Surg. 2010 Dec; 126(6): 2234–2242
Systematic reviews are a robust review of the evidence from relevant primary research studies.
The aim is to:
1. Identify all available research relevant to the review question.
2. Use explicit inclusion and exclusion criteria for studies to be accepted into the systematic review.
3. Apply established standards to critically appraise the quality of each study included in the review.
4. Extract and analyze the data from each included study. This may include a meta-analyses (a statistical analyses that combines the results of the multiple studies to identify common results and trends).
5. The results of the review should address the overall strength of evidence in support of the original question (i.e. weak, moderate, and strong).
Advantages:
Disadvantages:
Although the focus of this article has been to discuss quantitative study designs, we do not wish to underplay the importance of qualitative research. Qualitative research such as surveys, patient focus groups, interviews, telephone discussions can all help us to understand the patterns of health behaviors, capture illness experiences and give understanding to the beliefs and motivations of various groups of people. Qualitative research provides powerful insight into a problem and can inform hypotheses for quantitative research. Qualitative methods are a more holistic and person-centered way of uncovering thoughts and actions of human beings and definitely have a place in healthcare related research.
Understanding randomised controlled trials | Archives of Disease in Childhood (bmj.com)
Observational Studies: Cohort and Case-Control Studies - PMC (nih.gov)